myphysician360 is a virtual clinic! A virtual health clinic refers to the use of technology to provide healthcare for a patient who is oftentimes outside of a traditional healthcare setting.
Virtual health clinics have many benefits. You can access them from anywhere, they’re more affordable than a doctor’s visit or the ER, and you get to avoid exposure to other sick patients.
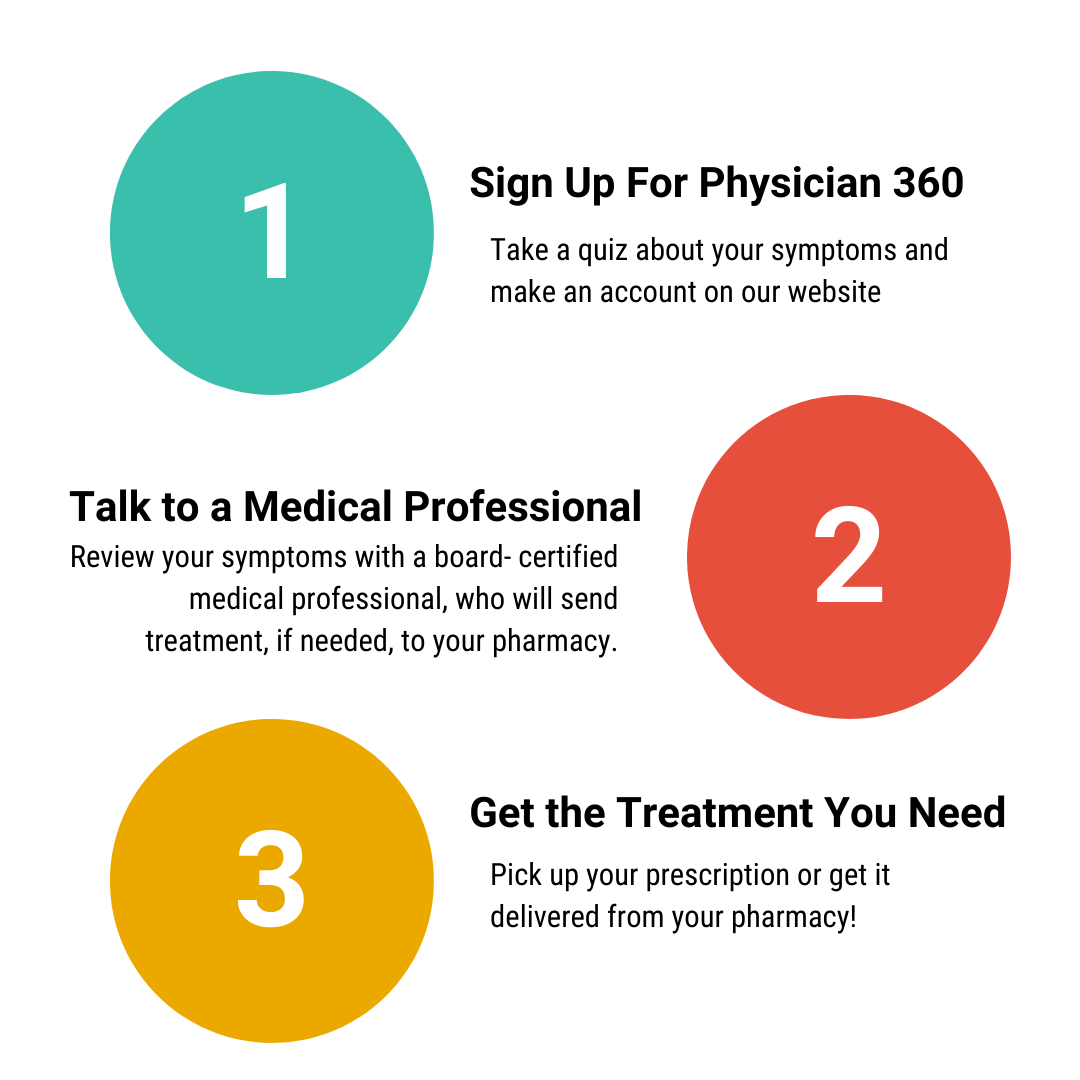
Welcome to myphysician360. Here’s how it works: Once you create an account with us and describe your symptoms, simply meet with one of our board-certified medical professionals in minutes and get the treatment you need, sent to a pharmacy of your choice.
Convenient, affordable, and easy care. This is myphysician360.